N-Iodosuccinimide (NIS)
N-Iodosuccinimide (NIS) is an iodinating agent that is used for various electrophilic iodinations and as source for iodine in radical reactions.
Recent Literature
Because of the popularity of coupling reactions, iodoarenes have become increasingly important in organic synthesis. Iodine reacts directly with only the most activated of aromatic substrates which has led to the development of iodonium donating reagents such as NIS1 and NIS in combination with Triflic acid2. More recently it has been discovered that NIS with a catalytic amount of Trifuoroacetic acid will regioselectively iodinate activated aromatic compounds3. Various methoxy- or methyl-substituted aromatic compounds were regioselectively iodinated with N-iodosuccinimide and and a catalytic amount of trifluoroacetic acid with excellent yields under mild conditions and short reaction times4.
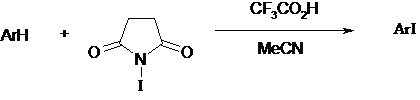
NIS has been used in combination with Triflic acid2 or BF3-H204 to iodinate deactivated arenes. This combination was very effective in iodinating various deactivated aromatics under mild conditions.
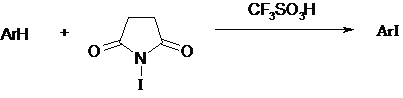
Using triethylamine as catalyst in Hunsdiecker reactions with N-Iodosuccinimide as the I+ source, cinnamic acids, and propiolic acids are converted to the corresponding α-halostyrenes and 1-halo-1-alkynes in good isolated yields within 1-5 min5.
J. Prakash, S. Roy, J. Org. Chem., 2002, 67, 7861-7864.
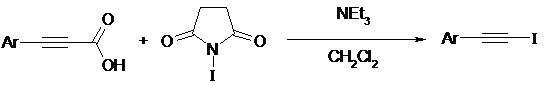
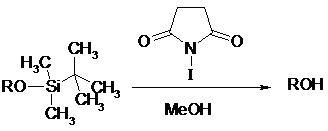
Various tert-butyldimethylsilyl ethers are easily removed in excellent yields by treatment with a catalytic amount of N-iodosuccinimide in methanol. This method allows a selective deprotection of TBDMS ethers of alcohols in the presence of TBDMS ethers of phenols6.
B. Karimi, A. Zamani, D. Zarayee, Tetrahedron Lett., 2004, 45, 9139-9141.
1.Carreno, M. C.; Ruano, J .L .G.; Sanz, G.: Toledo, M. A.; Urbano, A. Tetrahedron Lett. 1996, 37,4081.
2. Olah, G. A.; Qi, W.; Sandford, G;, Prakash, G. K. S. J. Org. Chem. 1993, 58, 3194.
3. Castanet, A.-S.; Colobert, F; P.-E. Broutin, P.-E. Tetrahedron Lett., 2002, 43, 5047-5048.
4. Prakash, G. S.; Mathew, T.; Hoole, D.; Esteves, P. M.; Wang, Q.; Rasul, G.; Olah, G. A. JACS, 2004, 126, 15770-15775.
5. Prakash, J.; Roy, S; J. Org. Chem., 2002, 67, 7861-7864
6. Karimi, B.; Zamani, A.; Zarayee, D. Tetrahedron Lett., 2004, 45, 9139-9141.
Related products:
t-Butyldimethylchlorosilane
Trifluoroacetic acid
Trifluoromethanesulfonic acid